Ozone water treatment
Ozone as a disinfectant in water treatment
Ozone is a gas molecule composed of three oxygen atoms (O3). It was first discovered in 1840 by a German chemist, and in 1906 it was used for the first time as a water disinfectant in a full-scale water treatment plant in France.
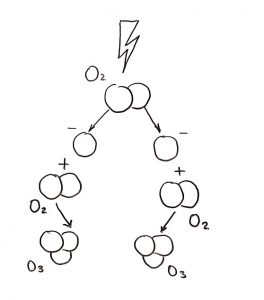
Ozone is generated by splitting a diatomic oxygen molecule (O2) into individual oxygen atoms. The radical oxygen then combines to another diatomic oxygen molecule to form ozone.
Ozone is a very strong oxidant. Its decomposition involves complex reactions and formation of hydroxyl radicals (•OH), and other radicals, such as hydroperoxyl radicals (HO2•), oxide radicals (O•), and ozonide radicals (O3•). The radicals formed play a significant role in the disinfection process, as they are stronger oxidants than the ozone itself.
Hydroxyl radicals are the most predominant and reactive specie, and the end product of all the reactions is molecular oxygen O2.
The effectiveness of the disinfection depends on the microorganism to be inactivated, the concentration of ozone and the contact time between ozone and the microorganism.
Ozone stability and half-life
The ozone molecule is unstable and highly reactive. It has a short half-life, ranging between a few minutes to a few hours. In distilled water, at a temperature of 20˚C, the half-life of ozone is around 25 minutes.
However, water quality conditions, such as temperature, pH, carbonate concentration, organic matter, and microorganisms, greatly affect ozone’s half-life. The half-life of ozone decreases with temperature and the concentration of organic constituents, while it increases with the addition of carbonates, which inhibit the reaction chain.
Generation of ozone
Because of its short half-life, ozone is generated on-site, during the water treatment process. It is generated using either Corona Discharge technology or UV light.
In Corona Discharge generators, a high-power alternating current is applied to air or oxygen that flow between two tubes or plates. The energy causes oxygen molecules to split and recombine with other oxygen molecules, to form ozone. The concentration of ozone produced depends on the source gas. When the source gas is air, ozone is produced at 0.5 – 3.0% by weight, while pure oxygen gas will form about twice the concentration.
The ozone is then fed into water by a diffuser, while the water is maintained in a contact chamber, in order to allow sufficient contact time with the contaminants. Remaining ozone must be either recycled or destroyed before being released to the atmosphere.
Ozone vs. Chlorine
In terms of cost, water treatment with ozone is more expensive than using chlorine.
However, unlike chlorine, disinfection with ozone does not leave a residual in the water. This has both advantages and disadvantages. On one hand, maintaining a residual is essential in order for the disinfectant to keep protecting the water distribution system. On the other hand, the chlorinated by-products may be harmful to health and to the environment.
In addition to not leaving a residual, disinfection with ozone has additional advantages:
- Disinfection with ozone is not dependent on pH.
- Ozone is more effective than chlorine at inactivating microorganisms (e.g., it is 100 times more effective than chlorine against Giardia).
- Greater effectiveness in oxidizing iron and manganese, that can be easily filtered out of the water.
- It is effective in reducing or eliminating taste and odor.
- Ozone doesn’t leave smell or taste.
- The use of ozone can reduce the need for using high levels of chlorine and other chemicals.
- By-products, such as THMs (trihalomethanes) and HAA (Haloacetic acids) are not formed.
The disinfection by-products formed as a result of using ozone include aldehydes, carboxylic acids, bromate, ketones and others.
Ct values (mg/L) for inactivation of Cryptosporidium by ozone
| Temperature, ˚C (˚F) | 2-log inactivation | 3-log inactivation |
|---|---|---|
| 10 (50) | 17.5 | 25.1 |
| 15 (59) | 9.6 | 13.8 |
| 20 (68) | 5.39 | 7.73 |
| 25 (77) | 3.08 | 4.42 |
Ct values (mg/L) for 2-log inactivation of Giardia by various disinfectants at 10˚C and pH 7
| Chlorine | Chlorine dioxide | Ozone |
|---|---|---|
| 69 | 15 | 0.95 |