Roles of potassium in plants
Potassium is an essential plant nutrient, one of the three macro-elements required by plants in relatively large quantities – nitrogen, potassium and phosphorus (NPK). What are the roles of potassium in plants? How does potassium affect plant performance?
Potassium enhances crop yields and quality in different ways. For example, it increases sugar content in fruits, size of vegetable crop fruits, protein content in cereals, helps maintaining longer shelf life, improves plants’ resistance to diseases and to drought and more.
Roles of potassium in plants
Potassium is involved in many processes in plants, from water regulation, through production of energy.
- Regulates opening and closing of the stomata – In order to open the stomata, potassium is actively pumped into the guard cells (the cells that surround the stomata). As a result, the osmotic potential osmotic potential inside the cell goes down, and water enters. Stomata close when potassium is pumped out of the guard cells.
- Influences the photosynthesis process and respiration:
- Potassium affects gas exchange in plants. By regulating the opening and closing of the stomata it regulates exchange of CO2 and O2 with the atmosphere.
- Involved in the synthesis of ATP (Adenosine triphosphate), which all cells use for energy.
- Regulates and improves water uptake – potassium that accumulates in root cells results in water entering into the plant root.
- Activates enzymes – The activation of many plant enzymes requires potassium. Potassium changes the three dimensional structure of the enzymes and, as a result, their rate of reaction and affinity for the substrate increase.
- Required for protein metabolism. Protein synthesis stops when there is no sufficient supply of potassium for the plant.
- Plants require potassium for proper uptake and use of other nutrients, such as nitrate (NO3–). Potassium accompanies nitrate, as a counter-ion, as it translocates within the plant.
- Potassium strengthen cell walls.
Potassium deficiencies in plants
Different crops may display different deficiency symptoms. However, the most common visual symptom of potassium deficiency in plants include scorching and yellowing of leaf edges, while the inner side of the leaf remains green. Leaf edges eventually become brown and die.
Other deficiency symptoms include:
- Smaller leaves.
- Poor crop yield
- Poor yield quality – size, uniformity, sugar content, protein content etc.
- Shorter shelf life.
- The crop might be more susceptible to diseases.
 Potassium deficiency on soybean
Potassium deficiency on soybean
Potassium deficiency in corn
Potassium deficiency in banana seedlings
Potassium availability for plants
Plants take up potassium from the soil solution in its elemental form K+.
The availability of potassium for plants is mainly dependent on soil composition and properties and on cultural practices. Heavy, clay soils, have a higher cation exchange capacity (CEC) and, therefore, retain more available potassium than light, sandy soils.
Acidic soils also have lower CEC, because H+ ions occupy the exchange sites on soil clay particles. As a result, less potassium is available for the plants.
Potassium in soil
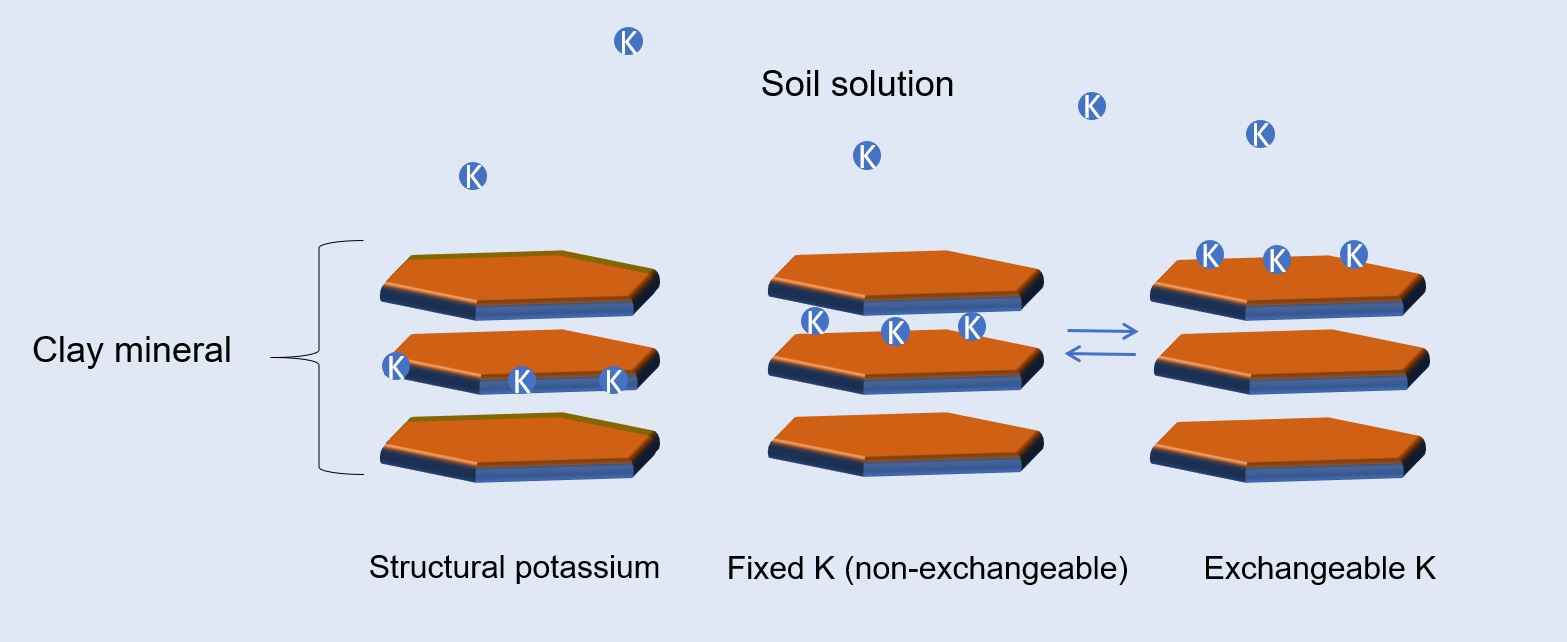
Potassium forms in soil can be classified to four categories:
- Mineral, or structural potassium
- Non-exchangeable, fixed potassium
- Exchangeable potassium
- Potassium in soil solution
Potassium availability to plants is the basis for the classification. The availability of potassium to plants may vary, depending on the type of soil and environmental conditions.
Structural potassium – Potassium is a constituent of soil minerals and is bonded within the crystalline structure of minerals, such as feldspars, clay minerals and micas in the soil. Plants cannot absorb structural potassium . However, small quantities are slowly released to the soil solution by long chemical weathering processes.
Fixed potassium – Potassium can become trapped (“fixed”) between the structural layers of clay minerals layers. This occurs mainly during wetting and drying cycles of the soil. Because some of the fixed potassium is in equilibrium with the exchangeable potassium, small quantities of fixed potassium can become slowly available to plants throughout the growing season.
Exchangeable potassium – The negatively charged surfaces of clay minerals and organic matter adsorb potassium, as it carries a positive charge. The exchangeable potassium is in equilibrium with the soil solution. Therefore, potassium depleted from the soil solution, as a result of plant uptake, can be easily replenished by the exchangeable potassium. As a result, this form of potassium is readily available for plants and is considered the most important pool of potassium.
Potassium in the soil solution – The soil solution is the immediate pool of nutrients for plants. Potassium dissolved in the soil solution is, therefore, readily available for plants. However, this pool of potassium is small and does not usually represent the amount of potassium available to plants.
Common potassium fertilizers
Various types of potassium fertilizers are available. All potassium sources are soluble. However, some fertilizers may contain insoluble compounds, such as iron oxide.
Potassium chloride (Muriate of potash, MOP):
- Formula: KCl
- Composition: 60% K2O (50% K) and 45% Cl–.
- A highly soluble potassium fertilizer.
- Solubility ranges from 275 g/liter at 30°C and 229 g/liter at 5°
- Should not be applied to crops that chloride-sensitive or to seeds.
- Most economic source of potassium for plants.
Potassium nitrate:
- Formula: KNO3
- Composition: 13% nitrate nitrogen and 46% K2O (38% K)
- Very soluble
- Solubility ranges from 458 g/liter at 30°C and 133 g/liter at 5°
- Serves also as a source of nitrogen for plants
- Has a relatively high cost
- Used mainly for greenhouse crops and in hydroponics
An example of a potassium nitrate fertilizer here.
Potassium sulfate (Sulfate of potash, SOP):
- Formula: K2SO4
- Composition: 52% as K20 (43% K) and 54% SO42- (18% S)
- Has a relatively low solubility
- Solubility ranges from 120 g/liter at 25°C and 80 g/liter at 5°
- Mainly used for chloride-sensitive plants and when sulfur fertilization is required
Mono potassium phosphate (MKP):
- Formula: KH2PO4
- Composition: 34% K20 (28% K) and 52% P2O5 (22.5% P)
- Solubility ranges from 300 g/liter at 25°C and 110 g/liter at 5°
Additional types of fertilizers that contain potassium are available, mainly compound fertilizers that are composed of the above straight fertilizers. These fertilizers contain three plant nutrients or more, mostly nitrogen, potassium and phosphorus..
Furthermore, a research by the MIT suggests that potassium fertilizers can be produced from feldspar rocks.
Fertilization and Irrigation – Theory and Best Practices. 2021 Edition by Guy Sela. Download today.