How to calculate nutrient solution formulas
A nutrient solution is the mix of irrigation water with added nutrients. It is mainly used in soilless culture and hydroponics, where the nutrient solution is the only source of nutrients to the plants.
Calculating and balancing the nutrient solution formula might be confusing. Following the proper steps is the best way to avoid mistakes. These steps are:
Step 1 - Test your source water
The source water may contain essential nutrients, which must be accounted for. Therefore, make sure to test your irrigation water before calculating the fertilizers to be added to the nutrient solution.
| Water source | Recommended tests |
| Groundwater | EC, pH, K, Ca, Mg, SO4, B, HCO3, Na, Cl, Fe |
| Surface water | EC, pH, Ca, Mg, HCO3, Na, Cl |
| Desalinated water | EC, pH, Ca, Mg, SO4, B, HCO3, Na, Cl |
Note that the lab may provide the test results in different units, such as ppm, mg/l, meq/l, mmol/l.
To simplify the calculation, convert all values to ppm. A conversion table is available here.
Agronomy Toolkit – Free Download
Are you looking for helpful resources to improve your agronomic practices? This FREE download includes essential information such as unit conversions, soil and water analysis interpretation, and more. Simply click the button below to access the toolkit and start improving your agronomic knowledge today. Don’t miss out on this valuable resource!
Step 2 – Obtain the nutrient requirements of the crop
Determine the nutrient rates your crop requires. You can use recommendations from the literature, or
your own experience.
If nutrient requirements are provided in units other than ppm or mg/l, it is recommended to convert
the values to ppm (1ppm = 1 mg/l).
Step 3 – Calculate the amount of fertilizer needed to provide the required nutrients.
To do so, simply deduct the source water test results from the nutrient requirements of the crop. Repeat that for each nutrient.
For example, if magnesium requirement is 60 ppm and the source water contains 40 ppm, then 60-40=20 ppm of magnesium has to be added with fertilizers. This means 20 milligrams of magnesium have to be added to each liter of nutrient solution.
A negative value means your source water contains more of the nutrient than the crop needs and, therefore, you should not use fertilizers that contain that nutrient.
Step 4 – Make a list of your available fertilizers
The fertilizer you select must contain all nutrients that need to be added, as per the calculation in step 3.
Check which fertilizers you have available that contain those nutrients.
Step 5 – Calculate fertilizer rates
Begin with the fertilizer that contains a unique nutrient, that other fertilizers do not contain. For example, if the only available source of calcium you have is calcium nitrate, start the calculation with this fertilizer.
For solid fertilizers:
FR = 100 x NA / %N
Where
FR is the fertilizer rate to apply in ppm (or mg/l).
NA is the required nutrient concentration to be added by this fertilizer in ppm (1 ppm = mg/l = 1 g/m3).
N is the concentration of the nutrient in the fertilizer.
For liquid fertilizers:
FR = NA / (% N x D x 10)
Where D is the density of the fertilizer in kg/L and FR is the fertilizer rate in of ml/L or L/m3.
And when the density of the fertilizer (D) is given in lbs/gallon, the formula becomes:
FR = NA / (%N x D x 11.98)
Where FR is the required application rate in gal/1000gal.
The percent nutrients that a fertilizer contains is usually given as a percent weight to weight and, therefore, dividing by the density of the fertilizer is required.
Example:
Calculate the rate of magnesium sulfate (a solid fertilizer) , required to apply 20 ppm of magnesium (Mg).
Magnesium sulfate contains 9.1 % Mg (Magnesium) and 14% S (sulfur).Therefore:
NA = 20 mg/l
N = 9.1
FR = 100 x 20 / 9.1 = 220 mg/l
Therefore, adding 220 mg/l of magnesium sulfate to the nutrient solution would contribute 20 ppm of magnesium.
Using the same formula, we can calculate the rate of sulfur added by this fertilizer:
220 = 100 x NA / 14
NA = 220 X 14 / 100 =30.8 ppm = 30.8 mg/L sulfur
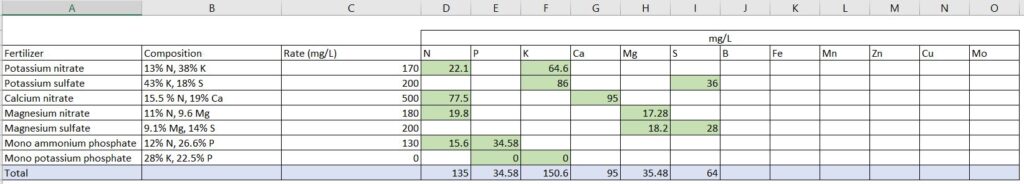
Step 6: Repeat the calculation to balance all nutrients
Now, you have to find the right combination of fertilizers that balances all the required nutrients.
Since for some nutrients you may have to use multiple fertilizer sources, it is recommended to prepare an Excel table that includes the fertilizers to be used and the rate of nutrients each of them contributes.
Some key points to keep in mind
- The concentration of the nutrient in the fertilizer may be given in the oxide form. Pay attention for which form you do the calculation. For example, MAP 0-52-34 contains 52% K2O and 34% P2O5. To calculate the rate of the fertilizer required to apply certain concentrations of K and P, you should first convert % K2O and P2O5 % of K and P.52% P2O5 = 22.7% P
38% K2O = 31.6% K
Click here to view a conversion table
- Nitrogen can be applied in two different forms – nitrate (NO3–) and ammonium (NH4+). The ratio between these two forms is of a great importance in nutrient solutions. Therefore, you may want to break down the nitrogen calculation to these two forms. For example, MAP (mono ammonium phosphate) contains 12% N-NH4, while potassium nitrate contains 13% N-NO3. This means the nitrogen in MAP is in its ammonium form, while in potassium nitrate it is in the nitrate form. Some fertilizers may contain both forms.
- Lowering the pH of the nutrient solution is often necessary. You can do this by adding acid to the nutrient solution. The three most common acids are nitric acid, phosphoric acid and sulfuric acid. As their name suggests, these acids contribute nutrients to the nutrient solution. Nitric acid contributes nitrogen, phosphoric acid – phosphorus and sulfuric acid – sulfur. When making calculations, you should consider these added nutrients.
🌱 Struggling with Nutrient Solutions? 🤔
Unlock the key to perfect nutrient balance in hydroponic and soilless growing systems.
Say goodbye to deficiencies, imbalances, and inefficiencies – and start growing healthier, more productive plants today!

🌿 The Ultimate Guide for Hydroponic Growers & Soilless Cultivation
This practical, easy-to-follow book will teach you how to formulate precise nutrient solutions, prevent deficiencies, and optimize irrigation strategies for healthy, vigorous plant growth.
With “Fertilization and Irrigation – Theory and Best Practices,” you’ll learn:
- How to create and adjust nutrient solutions for hydroponic crops.
- The science behind nutrient uptake in soilless media.
- How to prevent common nutrient deficiencies & toxicities.
- The role of pH, EC, and water quality in plant nutrition.