What are urea fertilizers?
Urea fertilizers are widely used in agriculture. They are considered an economic nitrogen source.
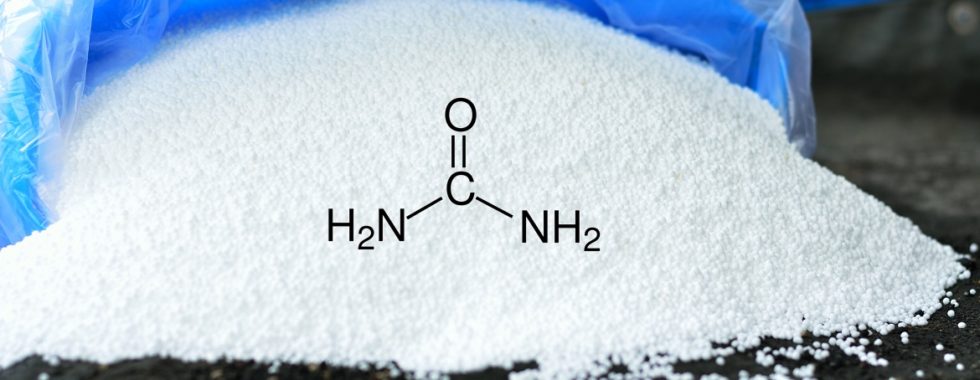
The chemical formula of urea is CO(NH2)2 and, in nature, urea is excreted in the urine of mammals. Commercial urea fertilizers are produced by reacting ammonia with carbon dioxide.
In its solid form, urea is provided as either prills or granules. Granules are slightly larger than prills and are more dense. Both prilled and granular urea fertilizers contain 46% N.
Nitrogen leaching and volatilization rates are usually higher when using the prilled form. Therefore, granular urea fertilizers are 15-20% more efficient than prilled.
Urea fertilizers are highly soluble (solubility of 1079 g/L at 20ºC). Therefore, in addition to soil applications, urea fertilizers can be also used in fertigation or as a foliar application. However, urea fertilizers should not be used in soilless culture, as urea will immediately leach out of the container.
The NPK grade of a solid urea fertilizer is 46-0-0. Another fertilizer containing high concentration of urea is Urea Ammonium Nitrate (UAN). UAN is a liquid fertilizer containing between 28 and 32% nitrogen. 50% of the nitrogen is urea, 25% ammonium nitrogen and 25% nitrate nitrogen.
REACTIONS OF UREA FERTILIZERS IN SOIL
Plants cannot absorb urea nitrogen. In order for the plant to absorb nitrogen applied as urea, nitrogen must be converted into ammonium (NH4+) and nitrate (NO3‑), which are the nitrogen forms that plants can use.
Once applied, the urea fertilizer reacts with water in the soil and with urease, an enzyme that exists abundantly in soils, and goes through the hydrolysis process, in which urea is converted into ammonium carbonate.
Ammonium carbonate is then converted into ammonium or to ammonia gas (NH3), depending on conditions such as pH, temperature and soil moisture.
Ammonia gas readily volatilizes from the soil and, as a result, significant losses of nitrogen may occur if conditions favor formation of ammonia rather than ammonium.
High soil pH and temperature result in greater losses of nitrogen.
Soil pH – High soil pH increases the rate of volatilization, as more ammonium is converted into ammonia gas.
Soil temperature – high soil temperatures increase the rate of urea hydrolysis, as it increases the activity of urease. At 70ºC urease becomes inactive due to denaturation.
The hydrolysis reaction is as follows:
(NH2)2CO + 2H2O –> (NH4)2CO3
(NH4)2CO3 + H2O –> 2NH3 + 2H2O + CO2
NH3 + H2 –> NH4+ +OH– (ammonification)
2NH4+ + 4O2 –> 2NO3– +4H++2H2O (nitrification)
As can be noted from the equation, the application of urea fertilizers results in an initial increase in the soil pH around the applied fertilizer, as hydrogen ions are consumed. However, the nitrification of ammonium to nitrate, which is carried out by soil bacteria, results in an a slightly acidifying net effect.
MOBILITY OF UREA FERTILIZERS IN SOIL
Since urea molecule is not electrically charged, it readily moves in soil. Leaching of nitrogen as urea will depend, therefore, on soil humidity and the time until urea hydrolysis is complete. Once urea is converted to ammonium, leaching reduces, because ammonium, which carries a positive charge, attracts to the negatively charged particles of the soil and, therefore, is relatively immobile.
Urea is more mobile than ammonium but a bit less mobile than nitrate.
Fertilization and irrigation
Get the book today. Both hard copy and digital version are available.
GUIDELINES FOR APPLICATION OF UREA FERTILIZERS
Urea fertilizers should be applied carefully. If not applied correctly, nitrogen losses due to volatilization may occur and, in some cases, urea might cause damage to germinating seeds.
Incorporate the urea fertilizer into the soil by irrigation or rainfall soon after its application. Avoid applying urea fertilizers to the soil surface without incorporating them into the soil results in greater losses of nitrogen. Losses are greater in soils of high pH.
Urea fertilizers should be applied when temperature is not too low or too high. Soil temperatures of 15- 20°C (70°F) are considered adequate.
Using urea fertilizers with urease inhibitors – urease inhibitors reduce the rate of hydrolysis and, therefore, of ammonia production and volatilization. This allows additional one or two weeks for incorporating the urea fertilizer into the soil by rain, irrigation or other means.
Urea fertilizers containing biuret – biuret is a chemical compound with the formula [H2NC(O)]2NH, which is formed in the manufacturing process of urea fertilizers. In high concentrations biuret might be toxic to crops.
Most urea fertilizers contain 1.0 to 1.3% biuret, which is considered safe to use. However, some crops are more sensitive. For foliar applications on sensitive crops, low-biuret fertilizers (contain approximately 0.25% biuret) should be used. Biuret might also damage seedlings if the urea fertilizer is placed too close to germinating seeds.