How to raise soil pH
Soil acidity is a major cause of reduced yields. Toxicity of micronutrients such as manganese, iron and aluminum may occur, while nutrients such as potassium, calcium and magnesium become unavailable. Furthermore, low soil pH impedes beneficial microbial activity in the soil.
Acidification can be caused by several factors. Some are natural causes and others are a result of an intensive agricultural activity.
Parent material – The rock from which the soil was formed affects its acidity. Soils developed from acidic parent material, such as granite, are likely to be more acidic than soils developed from calcareous parent material.
Rainfall – Excess rainfall leaches basic cations (calcium, magnesium, potassium) out from the soil. These cations are replaced by hydrogen, which makes soil more acidic.
Acid rain is precipitation that is more acidic than normal and, therefore it accelerates the acidification of soils. It contains sulfuric and nitric acids that are formed as a result of sulfur dioxide and nitrogen dioxide emissions from industry, vehicles and power-generating plants.
Decomposition of organic matter – The decomposition process of organic matter produces CO2 that reacts with water to form carbonic acid.
Application of certain types of fertilizers – The nitrification process of ammonium nitrogen yields hydrogen ions. Therefore, ammonium-based fertilizers have an acidifying effect. Sulfur fertilizers also have a strong acidifying effect.
Removal of basic nutrients with the harvest – Crops take up basic cations from soil and excrete protons in order to maintain electroneutrality. The basic cations are removed from the field at harvest and the soil acid neutralizing capacity is decreased.
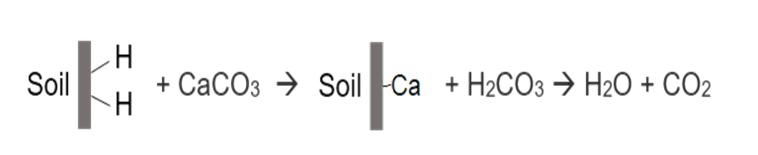
The most common method to raise soil pH is by applying agricultural lime (calcium carbonate) or equivalent liming materials. The reaction of lime in soil is represented by:
Once lime is added to an acidic soil, calcium replaces the exchangeable acidity, i.e. the hydrogen ions on the exchange complex, while the carbonate reacts with hydrogen ions in the soil solution to form carbon dioxide and water.
Because the optimum pH range varies among crops, the decision whether a lime application is required depends on the crop and the actual pH of the soil.
Example optimum pH range for several crops
| Crop |
Optimum soil pH |
| Alfalfa |
6.6-7.0 |
| Blueberries |
4.5-5.0 |
| Corn |
5.8-6.2 |
| Sweet potatoes |
5.0-5.5 |
| Peanut |
5.0-7.5 |
| Asparagus |
5.5-7.5 |
| Beets |
5.5-7.5 |
| Tomato |
6.0-6.5 |
| Soybeans |
6.6-7.0 |
| Sugar beets |
6.5-7.0 |
The process of raising soil pH to the desired level may take two to three years. Lime is applied to the soil surface before planting and should preferably be incorporated into the soil.
Amount of lime to apply
The amount of lime to apply depends on the ability of the soil to resist changes in pH (or buffering capacity). Labs measure a parameter known as the ‘buffer pH’. A buffer solution with a pH of 7.5 is added to the soil sample and the pH of the mix is reported on the soil test report.
A high buffer pH indicates that the buffering capacity of the soil is low (the addition of the buffer solution increased the pH considerably) and vice versa. The higher the buffer pH is, the higher the lime application rate should be.
The following equation can be used to calculate the lime requirement:
Lime requirement (lbs/acre) = 2000 X [ EA X [(target pH – soil pH)/(6.6- soil pH)]
Where EA is the exchangeable acidity.
If not given in the soil test report, the exchangeable acidity can be estimated from the buffer pH:
| Target pH | Exchangeable acidity |
| >=7 | 62.6- (2.33 x soil pH) – (7.13 x buffer pH) |
| >=6.5 | 51.8- (2.77 x soil pH) – (5.38 x buffer pH) |
| up to 6 | 39.3- (3.69 x soil pH) – (2.83 x buffer pH) |
Example:
Soil pH: 4.5
Target pH: 6.5
Buffer pH: 7.1
Exchangeable acidity = 51.8 – (2.77 x 4.5) – (5.38 x 7.1) = 1.137
Lime requirement = 2000 X [ EA X [(target pH – soil pH)/(6.6- soil pH)] =
2000 x [1.137 x (6.5-4.5)/(6.6-4.5) = 2165 lbs/acre
Raising soil pH using potassium carbonates
Potassium carbonate (K2CO3) and potassium bicarbonate (KHCO3) are water soluble and, therefore can be applied through drip irrigation systems. They can be used when it is necessary to raise soil pH in the rhizosphere and lime cannot be logistically applied.
Due to their high potassium content, the contribution of potassium must be accounted for in the fertilizer program. Ammonium fertilizers should be avoided, because the ammonium is converted to ammonia which might volatilize.